examples.elphf.phaseDiffusion¶
This example combines a phase field problem, as given in
examples.elphf.phase, with a binary diffusion problem, such as
described in the ternary example examples.elphf.diffusion.mesh1D, on a
1D mesh
>>> from fipy import CellVariable, FaceVariable, Grid1D, TransientTerm, DiffusionTerm, ImplicitSourceTerm, PowerLawConvectionTerm, Viewer
>>> from fipy.tools import numerix
>>> nx = 400
>>> dx = 0.01
>>> L = nx * dx
>>> mesh = Grid1D(dx = dx, nx = nx)
We create the phase field
>>> phase = CellVariable(mesh=mesh, name='xi', value=1., hasOld=1)
>>> phase.mobility = 1.
>>> phase.gradientEnergy = 0.025
>>> def pPrime(xi):
... return 30. * (xi * (1 - xi))**2
>>> def gPrime(xi):
... return 4 * xi * (1 - xi) * (0.5 - xi)
and a dummy electrostatic potential field
>>> potential = CellVariable(mesh = mesh, name = 'phi', value = 0.)
>>> permittivityPrime = 0.
We start with a binary substitutional system
>>> class ComponentVariable(CellVariable):
... def __init__(self, mesh, value = 0., name = '',
... standardPotential = 0., barrier = 0.,
... diffusivity = None, valence = 0, equation = None,
... hasOld = 1):
... self.standardPotential = standardPotential
... self.barrier = barrier
... self.diffusivity = diffusivity
... self.valence = valence
... self.equation = equation
... CellVariable.__init__(self, mesh = mesh, value = value,
... name = name, hasOld = hasOld)
...
... def copy(self):
... return self.__class__(mesh = self.mesh,
... value = self.value,
... name = self.name,
... standardPotential =
... self.standardPotential,
... barrier = self.barrier,
... diffusivity = self.diffusivity,
... valence = self.valence,
... equation = self.equation,
... hasOld = 0)
consisting of the solvent
>>> solvent = ComponentVariable(mesh = mesh, name = 'Cn', value = 1.)
and the solute
>>> substitutionals = [
... ComponentVariable(mesh = mesh, name = 'C1',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.3/.7) - numerix.log(.7/.3))]
>>> interstitials = []
>>> for component in substitutionals:
... solvent -= component
The thermodynamic parameters are chosen to give a solid phase rich in the solute and a liquid phase rich in the solvent.
>>> solvent.standardPotential = numerix.log(.7/.3)
>>> solvent.barrier = 1.
We create the phase equation as in examples.elphf.phase
and create the diffusion equations for the different species as in
examples.elphf.diffusion.mesh1D. The initial residual of the
diffusion equations is much larger than the norm of the right-hand-side
vector, so we use “initial” tolerance scaling for those equations
>>> def makeEquations(phase, substitutionals, interstitials):
... phase.equation = TransientTerm(coeff = 1/phase.mobility) \
... == DiffusionTerm(coeff = phase.gradientEnergy) \
... - (permittivityPrime / 2.) \
... * potential.grad.dot(potential.grad)
... enthalpy = solvent.standardPotential
... barrier = solvent.barrier
... for component in substitutionals + interstitials:
... enthalpy += component * component.standardPotential
... barrier += component * component.barrier
...
... mXi = -(30 * phase * (1 - phase) * enthalpy
... + 4 * (0.5 - phase) * barrier)
... dmXidXi = (-60 * (0.5 - phase) * enthalpy + 4 * barrier)
... S1 = dmXidXi * phase * (1 - phase) + mXi * (1 - 2 * phase)
... S0 = mXi * phase * (1 - phase) - phase * S1
...
... phase.equation -= S0 + ImplicitSourceTerm(coeff = S1)
...
... for Cj in substitutionals:
... CkSum = ComponentVariable(mesh = mesh, value = 0.)
... CkFaceSum = FaceVariable(mesh = mesh, value = 0.)
... for Ck in [Ck for Ck in substitutionals if Ck is not Cj]:
... CkSum += Ck
... CkFaceSum += Ck.harmonicFaceValue
...
... counterDiffusion = CkSum.faceGrad
... phaseTransformation = (pPrime(phase.harmonicFaceValue) \
... * Cj.standardPotential
... + gPrime(phase.harmonicFaceValue) \
... * Cj.barrier) * phase.faceGrad
... electromigration = Cj.valence * potential.faceGrad
... convectionCoeff = counterDiffusion + \
... solvent.harmonicFaceValue \
... * (phaseTransformation + electromigration)
... convectionCoeff *= \
... (Cj.diffusivity / (1. - CkFaceSum))
...
... Cj.equation = (TransientTerm()
... == DiffusionTerm(coeff=Cj.diffusivity)
... + PowerLawConvectionTerm(coeff=convectionCoeff))
...
... for Cj in interstitials:
... phaseTransformation = (pPrime(phase.harmonicFaceValue) \
... * Cj.standardPotential \
... + gPrime(phase.harmonicFaceValue) \
... * Cj.barrier) * phase.faceGrad
... electromigration = Cj.valence * potential.faceGrad
... convectionCoeff = Cj.diffusivity \
... * (1 + Cj.harmonicFaceValue) \
... * (phaseTransformation + electromigration)
...
... Cj.equation = (TransientTerm()
... == DiffusionTerm(coeff=Cj.diffusivity)
... + PowerLawConvectionTerm(coeff=convectionCoeff))
...
... for Cj in substitutionals + interstitials:
... Cj.solver = Cj.equation.getDefaultSolver(criterion="initial")
>>> makeEquations(phase, substitutionals, interstitials)
We start with a sharp phase boundary
or
>>> x = mesh.cellCenters[0]
>>> phase.setValue(1.)
>>> phase.setValue(0., where=x > L / 2)
and with a uniform concentration field \(C_1 = 0.5\) or
>>> substitutionals[0].setValue(0.5)
If running interactively, we create viewers to display the results
>>> if __name__ == '__main__':
... viewer = Viewer(vars=([phase, solvent]
... + substitutionals + interstitials),
... datamin=0, datamax=1)
... viewer.plot()
This problem does not have an analytical solution, so after iterating to equilibrium
>>> dt = 10000
>>> from builtins import range
>>> for i in range(5):
... for field in [phase] + substitutionals + interstitials:
... field.updateOld()
... phase.equation.solve(var = phase, dt = dt)
... for field in substitutionals + interstitials:
... field.equation.solve(var=field,
... dt=dt,
... solver=field.solver)
... if __name__ == '__main__':
... viewer.plot()
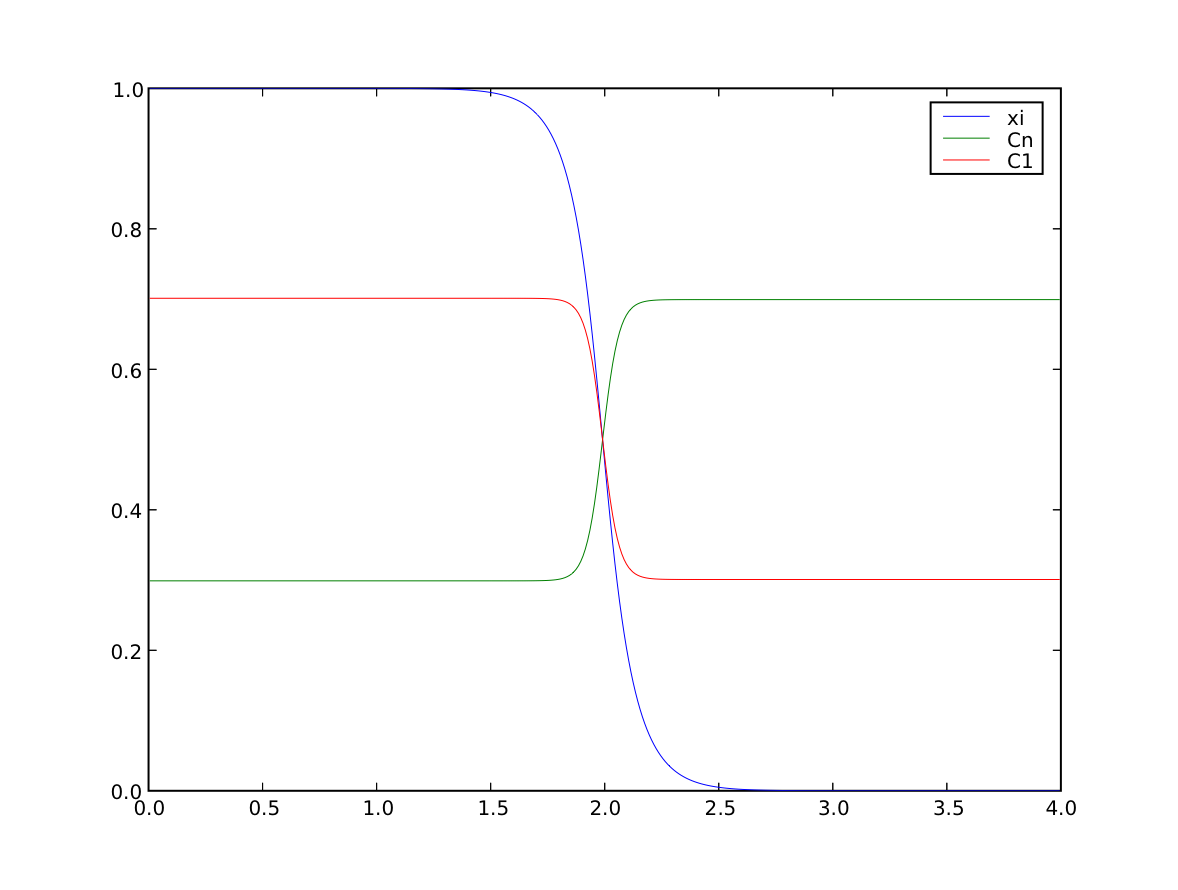
we confirm that the far-field phases have remained separated
>>> numerix.allclose(phase(((0., L),)), (1.0, 0.0), rtol = 1e-5, atol = 1e-5)
1
and that the solute concentration field has appropriately segregated into solute-rich and solute-poor phases.
>>> print(numerix.allclose(substitutionals[0](((0., L),)), (0.7, 0.3), rtol = 2e-3, atol = 2e-3))
1
The same system of equations can model a quaternary substitutional system as easily as a binary. Because it depends on the number of substitutional solute species in question, we recreate the solvent
>>> solvent = ComponentVariable(mesh = mesh, name = 'Cn', value = 1.)
and make three new solute species
>>> substitutionals = [
... ComponentVariable(mesh = mesh, name = 'C1',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.3/.4) - numerix.log(.1/.2)),
... ComponentVariable(mesh = mesh, name = 'C2',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.4/.3) - numerix.log(.1/.2)),
... ComponentVariable(mesh = mesh, name = 'C3',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.2/.1) - numerix.log(.1/.2))]
>>> for component in substitutionals:
... solvent -= component
>>> solvent.standardPotential = numerix.log(.1/.2)
>>> solvent.barrier = 1.
These thermodynamic parameters are chosen to give a solid phase rich in the solvent and the first substitutional component and a liquid phase rich in the remaining two substitutional species.
Again, if we’re running interactively, we create a viewer
>>> if __name__ == '__main__':
... viewer = Viewer(vars=([phase, solvent]
... + substitutionals + interstitials),
... datamin=0, datamax=1)
... viewer.plot()
We reinitialize the sharp phase boundary
>>> phase.setValue(1.)
>>> phase.setValue(0., where=x > L / 2)
and the uniform concentration fields, with the substitutional concentrations \(C_1 = C_2 = 0.35\) and \(C_3 = 0.15\).
>>> substitutionals[0].setValue(0.35)
>>> substitutionals[1].setValue(0.35)
>>> substitutionals[2].setValue(0.15)
We make new equations
>>> makeEquations(phase, substitutionals, interstitials)
and again iterate to equilibrium
>>> dt = 10000
>>> from builtins import range
>>> for i in range(5):
... for field in [phase] + substitutionals + interstitials:
... field.updateOld()
... phase.equation.solve(var = phase, dt = dt)
... for field in substitutionals + interstitials:
... field.equation.solve(var=field,
... dt=dt,
... solver=field.solver)
... if __name__ == '__main__':
... viewer.plot()
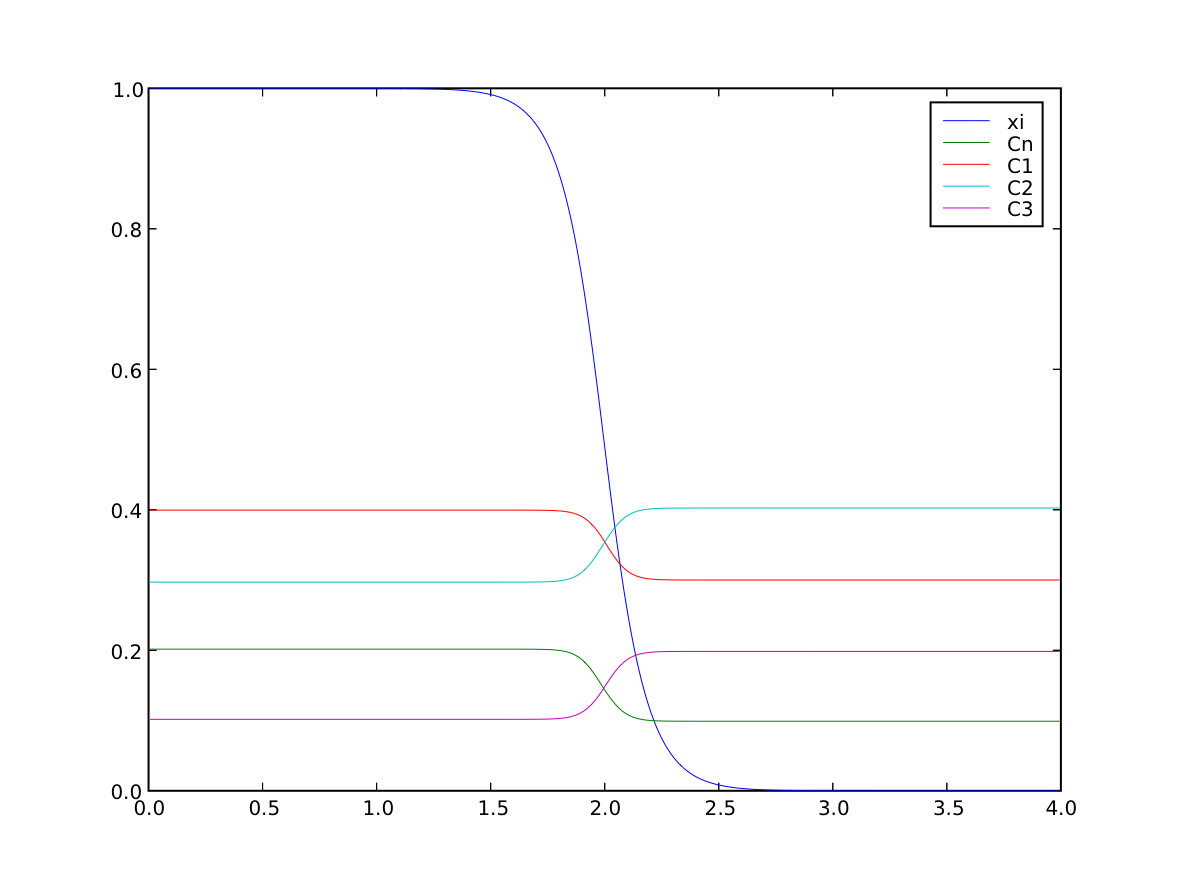
We confirm that the far-field phases have remained separated
>>> numerix.allclose(phase(((0., L),)), (1.0, 0.0), rtol = 1e-5, atol = 1e-5)
1
and that the concentration fields have appropriately segregated into their respective phases
>>> numerix.allclose(substitutionals[0](((0., L),)), (0.4, 0.3), rtol = 3e-3, atol = 3e-3)
1
>>> numerix.allclose(substitutionals[1](((0., L),)), (0.3, 0.4), rtol = 3e-3, atol = 3e-3)
1
>>> numerix.allclose(substitutionals[2](((0., L),)), (0.1, 0.2), rtol = 3e-3, atol = 3e-3)
1
Finally, we can represent a system that contains both substitutional and interstitial species. We recreate the solvent
>>> solvent = ComponentVariable(mesh = mesh, name = 'Cn', value = 1.)
and two new solute species
>>> substitutionals = [
... ComponentVariable(mesh = mesh, name = 'C2',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.4/.3) - numerix.log(.4/.6)),
... ComponentVariable(mesh = mesh, name = 'C3',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.2/.1) - numerix.log(.4/.6))]
and one interstitial
>>> interstitials = [
... ComponentVariable(mesh = mesh, name = 'C1',
... diffusivity = 1., barrier = 0.,
... standardPotential = numerix.log(.3/.4) - numerix.log(1.3/1.4))]
>>> for component in substitutionals:
... solvent -= component
>>> solvent.standardPotential = numerix.log(.4/.6) - numerix.log(1.3/1.4)
>>> solvent.barrier = 1.
These thermodynamic parameters are chosen to give a solid phase rich in interstitials and the solvent and a liquid phase rich in the two substitutional species.
Once again, if we’re running interactively, we create a viewer
>>> if __name__ == '__main__':
... viewer = Viewer(vars=([phase, solvent]
... + substitutionals + interstitials),
... datamin=0, datamax=1)
... viewer.plot()
We reinitialize the sharp phase boundary
>>> phase.setValue(1.)
>>> phase.setValue(0., where=x > L / 2)
and the uniform concentration fields, with the interstitial concentration \(C_1 = 0.35\)
>>> interstitials[0].setValue(0.35)
and the substitutional concentrations \(C_2 = 0.35\) and \(C_3 = 0.15\).
>>> substitutionals[0].setValue(0.35)
>>> substitutionals[1].setValue(0.15)
We make new equations
>>> makeEquations(phase, substitutionals, interstitials)
and again iterate to equilibrium
>>> dt = 10000
>>> from builtins import range
>>> for i in range(5):
... for field in [phase] + substitutionals + interstitials:
... field.updateOld()
... phase.equation.solve(var = phase, dt = dt)
... for field in substitutionals + interstitials:
... field.equation.solve(var=field,
... dt=dt,
... solver=field.solver)
... if __name__ == '__main__':
... viewer.plot()
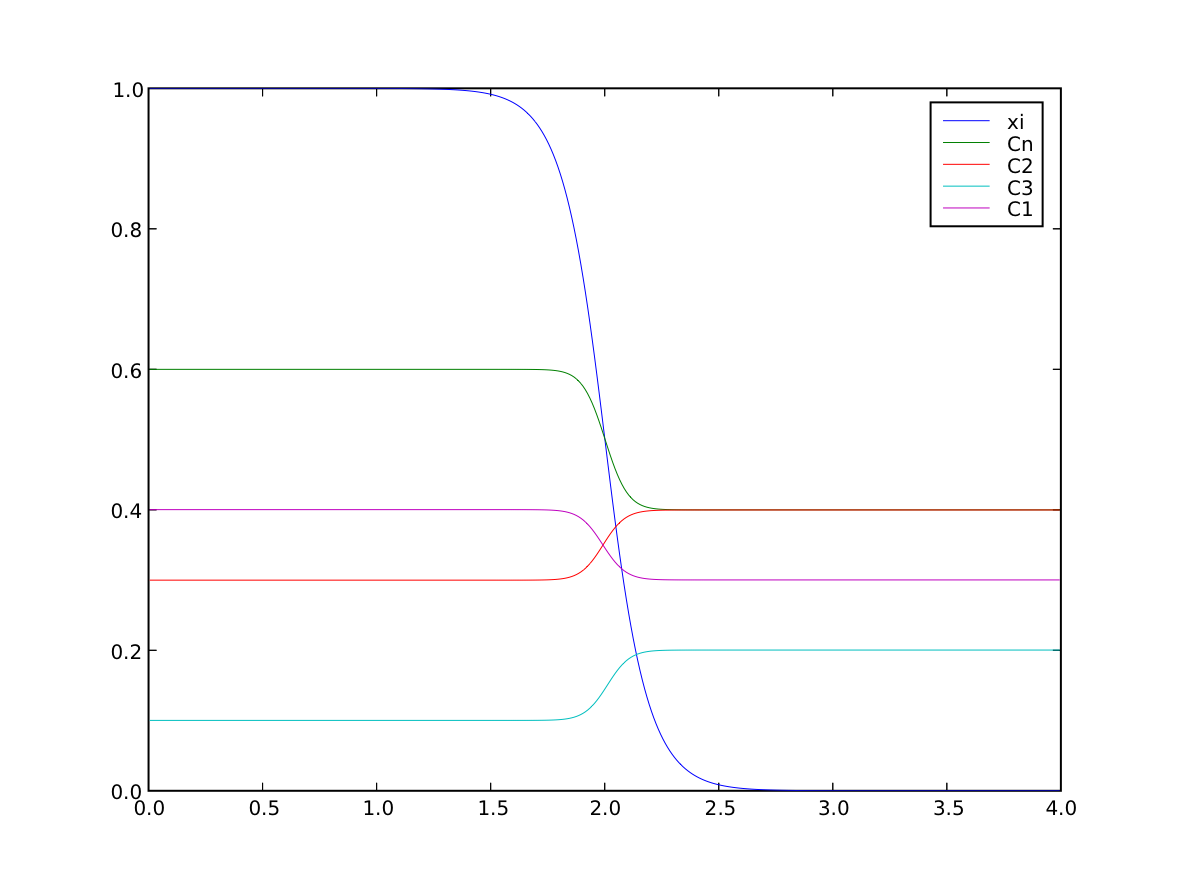
We once more confirm that the far-field phases have remained separated
>>> numerix.allclose(phase(((0., L),)), (1.0, 0.0), rtol = 1e-5, atol = 1e-5)
1
and that the concentration fields have appropriately segregated into their respective phases
>>> numerix.allclose(interstitials[0](((0., L),)), (0.4, 0.3), rtol = 3e-3, atol = 3e-3)
1
>>> numerix.allclose(substitutionals[0](((0., L),)), (0.3, 0.4), rtol = 3e-3, atol = 3e-3)
1
>>> numerix.allclose(substitutionals[1](((0., L),)), (0.1, 0.2), rtol = 3e-3, atol = 3e-3)
1
 FiPy
FiPy